Exploration of New Chemical Reactivities for Sustainable Molecular Transformations
Our future global sustainability mandates fundamental innovations in sciences and technologies with regard to resources, energy, and environment. Our lab is focusing on exploring new fundamental reactions that can drastically shorten synthetic steps, more directly transform renewable biomass and abundant feedstocks (CO2 and methane) into high valued products, and harvest solar light by chemical means and utilize photo-energy as energy input for chemical conversion.
Utilization of Natural Feedstocks
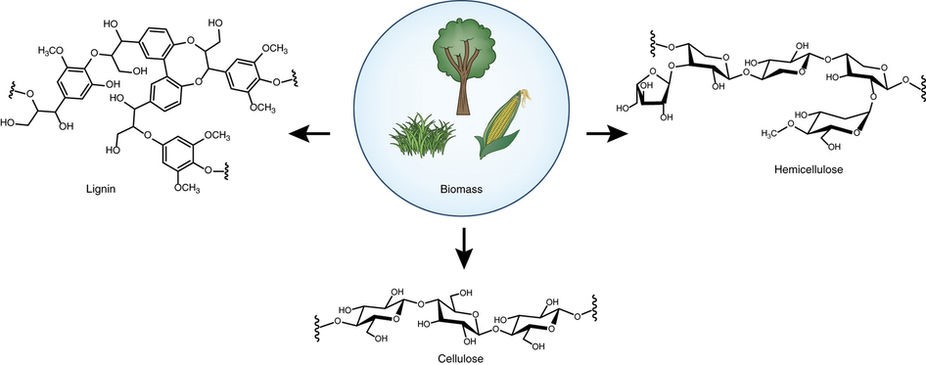
As fossil resources are undergoing fast depletion, the harvesting of sustainable aromatic compounds from biorenewable lignin has become highly appealing nowadays. Since lignin is the only source of renewable aromatic feedstock in nature, to realize the functionalization of lignin or the fine chemicals (e.g. phenols, phenyl ethers) derived from lignin is of paramount importance in sustainable transformations. Our group is dedicated to developing new strategies to activate phenols or diphenyl ethers by “hydrogen borrowing” and “dearomatization-rearomatization” strategies to realize a series of amination reactions to afford N-cyclohexyl amines and anilines.1 Besides, we have developed an efficient Cu/NHC oxidative system to catalyze aerobic cleavage of β-1 lignin models to aldehydes.2
(1) (a) Chen, Z.; Zeng, H.; Gong, H.; Wang, H.; Li, C. J. Palladium-Catalyzed Reductive Coupling of Phenols with Anilines and Amines: Efficient Conversion of Phenolic Lignin Model Monomers and Analogues to Cyclohexylamines. Chem. Sci. 2015, 6, 4174.
(b) Chen, Z.; Zeng, H.; Girard, S. A.; Wang, F.; Chen, N.; Li, C.-J. Formal Direct Cross-Coupling of Phenols with Amines. Angew. Chem. Int. Ed. 2015, 54, 14487.
(c) Qiu, Z.; Li, J.-S.; Li, C.-J. Formal Aromaticity Transfer for Palladium-Catalyzed Coupling between Phenols and Pyrrolidines/Indolines. Chem. Sci. 2017, 8, 6954.
(d) Zeng, H.; Cao, D.; Qiu, Z.; Li, C.-J. Palladium-Catalyzed Formal Cross-Coupling of Diaryl Ethers with Amines: Slicing the 4-O-5 Linkage in Lignin Models. Angew. Chem. Int. Ed. 2018, 57, 3752.
(e) Qiu, Z.; Lv, L.; Li, J.; Li, C.-C.; Li, C.-J. Direct Conversion of Phenols into Primary Anilines with Hydrazine Catalyzed by Palladium. Chem. Sci. 2019, 10, 4775.
(f) Zeng, H.; Qiu, Z.; Domínguez-Huerta, A.; Hearne, Z.; Chen, Z.; Li, C.-J. An Adventure in Sustainable Cross-Coupling of Phenols and Derivatives Via Carbon–Oxygen Bond Cleavage. ACS Catal. 2017, 7, 510.
(g) Cao, D.; Zeng, H.; Li, C.-J. Formal Cross-Coupling of Diaryl Ethers with Ammonia by Dual C(Ar)–O Bond Cleavages. ACS Catal. 2018, 8, 8873.
(h) Wang, Z.; Niu, J.; Zeng, H.; Li, C.-J. Construction of Spirocyclic Tetrahydro-Β-Carbolines Via Cross-Annulation of Phenols with Tryptamines in Water. Org. Lett. 2019, 21, 7033.
(i) Dominguez-Huerta, A.; Perepichka, I.; Li, C.-J. Direct Synthesis of Diphenylamines from Phenols and Ammonium Formate Catalyzed by Palladium. ChemSusChem 2019, 12, 2999.
(2) Zhou, Z.-z.; Liu, M.; Li, C.-J. Selective Copper–N-Heterocyclic Carbene (Copper-Nhc)-Catalyzed Aerobic Cleavage of Β-1 Lignin Models to Aldehydes. ACS Catal. 2017, 7, 3344.
Light-Mediated Innovations in Green Chemistry
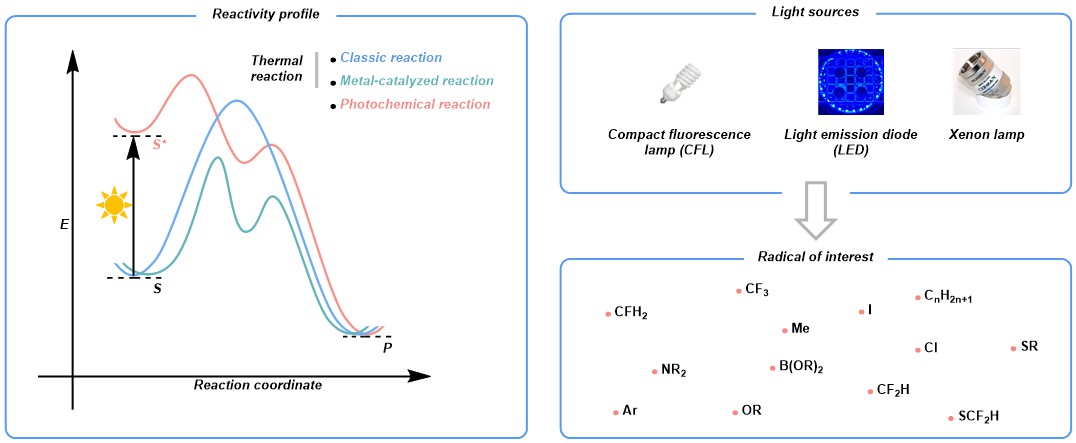
Photons are unique “reagents” in organic chemistry, ubiquitous in nature and readily accessible in the laboratory thanks to the advancement of light technologies. With irradiation, chemical entities become excited, featuring a markedly different reaction profile compared to its ground state. Nowadays, this radical-based one-electron paradigm is experiencing a renaissance and continuously reshaping people's thoughts on reaction design and compound synthesis due to its unparalleled ability in creating numerous open-shell species for chemical transformations. In this context, our group is interested in developing protocols to use light to empower the generation of radicals and furnish a series of products that find broad application in various fields. In contrast to many others, we focus primarily on metal catalyst-free methods by strategically designing and enabling reactions with complementary reactivity in the absence of noble metals.
1) Liu, W.; Li, J.; Huang, C.-Y., Li, C.-J. Aromatic Chemistry in the Excited State: Facilitating Metal-Free Substitutions and Cross-Couplings. Angew. Chem. Int. Ed. 2019, 58, 2-13.
2) Li, C.-J. Exploration of New Chemical Reactivities for Sustainable Molecular Transformations. Chem. 2016, 423-437.
The New Era of Umpolung Reactions and Hydrazone Chemistry
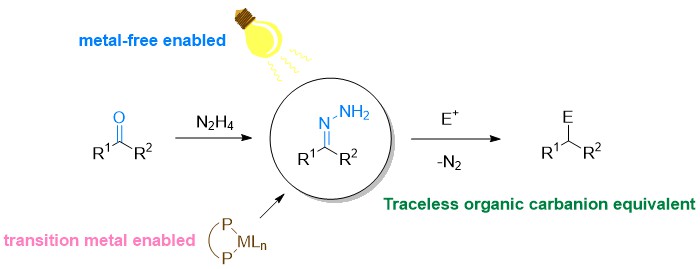
The Wolff–Kishner reduction, discovered in the early 1910s, is a fundamental and effective tool to convert carbonyls into methylenes via deoxygenation under strongly basic conditions. For over a century, numerous valuable chemical products have been synthesized by this classical method. The reaction proceeds via the reversible formation of hydrazone followed by deprotonation with the strong base to give an N-anionic intermediate, which affords the deoxygenation product upon denitrogenation and protonation. By examining the mechanistic pathway of this century old classical carbonyl deoxygenation, we envisioned and subsequently developed two unprecedented new types of chemical transformations: a) alcohol deoxygenation and b) C–C bond formations with various electrophiles including Grignard-type reaction, conjugate addition, olefination, and diverse cross-coupling reactions.1-11
(1) Chen, N.; Dai, X.-J.; Wang, H.; Li, C.-J. Umpolung Addition of Aldehydes to Aryl Imines. Angew. Chem. Int. Ed. 2017, 56, 6260-6263.
(2) Dai, X.-J.; Wang, H.; Li, C.-J. Carbonyls as Latent Alkyl Carbanions for Conjugate Additions. Angew. Chem. Int. Ed. 2017, 56, 6302-6306.
(3) Wang, H.; Dai, X.-J.; Li, C.-J. Aldehydes as Alkyl Carbanion Equivalents for Additions to Carbonyl Compounds. Nat. Chem. 2017, 9, 374-378.
(4) Wei, W.; Dai, X.-J.; Wang, H.; Li, C.; Yang, X.; Li, C.-J. Ruthenium(II)-Catalyzed Olefination via Carbonyl Reductive Cross-Coupling. Chem. Sci. 2017, 8, 8193-8197.
(5) Lv, L.; Qiu, Z.; Li, J.; Liu, M.; Li, C.-J. N2H4 as Traceless Mediator for Homo- and Cross- Aryl Coupling. Nat. Commun. 2018, 9, 4739.
(6) Lv, L.; Zhu, D.; Tang, J.; Qiu, Z.; Li, C.-C.; Gao, J.; Li, C.-J. Cross-Coupling of Phenol Derivatives with Umpolung Aldehydes Catalyzed by Nickel. ACS Catal. 2018, 8, 4622-4627.
(7) Tang, J.; Lv, L.; Dai, X.-J.; Li, C.-C.; Li, L.; Li, C.-J. Nickel-Catalyzed Cross-Coupling of Aldehydes with Aryl Halides via Hydrazone Intermediates. Chem. Commun. 2018, 54, 1750-1753.
(8) Zhu, D.; Lv, L.; Li, C.-C.; Ung, S.; Gao, J.; Li, C.-J. Umpolung of Carbonyl Groups as Alkyl Organometallic Reagent Surrogates for Palladium-Catalyzed Allylic Alkylation. Angew. Chem. Int. Ed. 2018, 57, 16520-16524.
(9) Li, C.-J.; Huang, J.; Dai, X.-J.; Wang, H.; Chen, N.; Wei, W.; Zeng, H.; Tang, J.; Li, C.; Zhu, D.; Lv, L. An Old Dog with New Tricks: Enjoin Wolff–Kishner Reduction for Alcohol Deoxygenation and C–C Bond Formations. Synlett. 2019, 30, 1508-1524.
(10) Lv, L.; Zhu, D.; Li, C.-J. Direct Dehydrogenative Alkyl Heck-Couplings of Vinylarenes with Umpolung Aldehydes Catalyzed by Nickel. Nat. Commun. 2019, 10, 715.
(11) Dai, X.-J.; Li, C.-J. En Route to a Practical Primary Alcohol Deoxygenation. J. Am. Chem. Soc. 2016, 138, 5433-5440.
Merging semiconductor technologies: solar-powered nano-catalyst for unprecedented conversion
Using the cutting-edge nano-semiconductor manufacturing technology (molecular beam epitaxy), a micro-array of photosensitizing nanowire can be synthesized based on gallium nitride (GaN), a highly robust material with melting point over 2500 °C. The nano-catalyst achieves record conversion efficiency in the fixation of atmospheric nitrogen into ammonia, and the dehydration of methanol into methyl carbene.
Cross-Dehydrogenative Coupling (CDC)
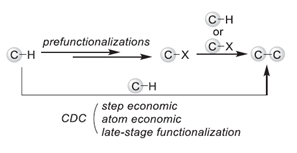 Cross-Dehydrogenative Coupling (CDC), also called Dehydrogenative Cross-Coupling or oxidative C-H/C-H cross coupling, refers to the formation of C-C bond from two C-H bonds directly via formal removal of an equivalent of H2. It is a class of reaction proposed by our group based on the concept and ideal of Green Chemistry. Since the CDC coupling can construct the C-C bond with unfunctionalized C-H species and eliminate the need for the preparation of reaction substrates, the CDC reaction has the advantages of high efficiency, atomic economy, and environmental friendliness. We endeavor to develop new types of CDC reactions to construct bonds between various kinds of C-H bonds, under metal-catalyzed, metal-free, photocatalytic, electrocatalytic, or thermal reaction conditions.
Cross-Dehydrogenative Coupling (CDC), also called Dehydrogenative Cross-Coupling or oxidative C-H/C-H cross coupling, refers to the formation of C-C bond from two C-H bonds directly via formal removal of an equivalent of H2. It is a class of reaction proposed by our group based on the concept and ideal of Green Chemistry. Since the CDC coupling can construct the C-C bond with unfunctionalized C-H species and eliminate the need for the preparation of reaction substrates, the CDC reaction has the advantages of high efficiency, atomic economy, and environmental friendliness. We endeavor to develop new types of CDC reactions to construct bonds between various kinds of C-H bonds, under metal-catalyzed, metal-free, photocatalytic, electrocatalytic, or thermal reaction conditions.
References:
- Huang, C.-Y.; Li, J.; Liu, W.; Li, C.-J. Diacetyl as a “traceless” visible light photosensitizer in metal-free cross- dehydrogenative coupling reactions. Sci. 2019, 10, 5018-5024.
- Han, W.-J.; Pu, F.; Li, C.-J.; Liu, Z.-W.; Fan, J.; Shi, X.-Y. Carboxyl-Directed Conjugate Addition of C−H Bonds to α,β-Unsaturated Ketones in Air and Water. Synth. Catal. 2018, 360, 1358−1363.
- Zhi, H.; Ung, S. P.-M.; Liu, Y.; Zhao, L.; Li, C.-J. Phosphorylation of Glycine Derivatives via Copper(I)-Catalyzed Csp3−H Bond Functionalization. Adv. Synth. Catal. 2016, 358, 2553−2557.
- Gong, H.; Zeng, H.; Zhou, F.; Li, C.-J. Rhodium(I)-Catalyzed Regiospecific Dimerization of Aromatic Acids: Two Direct C−H Bond Activations in Water. Chem., Int. Ed. 2015, 54, 5718−5721.
- Li, Z.; Cao, L.; Li, C.-J. FeCl2-Catalyzed Selective C−C Bond Formation by Oxidative Activation of a Benzylic C−H Bond. Angew. Chem., Int. Ed. 2007, 46, 6505−6507.
- Zhang, Y.; Li, C.-J. Highly Efficient Cross-Dehydrogenative- Coupling between Ethers and Active Methylene Compounds. Angew. Chem., Int. Ed. 2006, 45, 1949−1952.
- Zhang, Y.; Li, C.-J. DDQMediated Direct Cross-Dehydrogenative-Coupling (CDC) between Benzyl Ethers and Simple Ketones. J. Am. Chem. Soc. 2006, 128, 4242− 4243.
- Li, Z.; Bohle, D. S.; Li, C.-J. Cu-catalyzed crossdehydrogenative coupling: A versatile strategy for C−C bond formations via the oxidative activation of sp3 C−H bonds. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 8928.
- Li, Z.; Li, C.-J. Highly Efficient Copper-Catalyzed Nitro- Mannich Type Reaction: Cross-Dehydrogenative-Coupling between sp3 C−HBond and sp3 C−HBond. J. Am. Chem. Soc. 2005, 127, 3672− 3673.
- Li, Z.; Li, C.-J. CuBr-Catalyzed Efficient Alkynylation of sp3 C− H Bonds Adjacent to a Nitrogen Atom. J. Am. Chem. Soc. 2004, 126, 11810−11811